Summary
While urban areas benefit from treated and regulated public water supplies, rural communities often depend on untreated sources like wells, boreholes, and rainwater. Access to clean drinking water is essential for health, yet many New Zealanders who rely on private water sources face a higher risk of waterborne diseases such as Campylobacter infection. This Briefing outlines our recently published findings showing the elevated risks for those using private water supplies, particularly in areas with a higher density of dairy farming. These findings support regulatory action to protect those at higher risk of campylobacteriosis in rural areas, notably extending the National Environmental Standards for sources of Drinking Water to include private supplies.
Microbial pathogens are a major threat to the safety of drinking water. These harmful organisms, which can cause waterborne illnesses, include bacteria such as Campylobacter, E. coli, Salmonella, Shigella, and Yersinia, as well as protozoa like Giardia and Cryptosporidium. Enteric viruses like norovirus, rotavirus, and hepatitis A are shed in the feces of infected individuals and can also contaminate water sources, leading to fecal-oral transmission.1
Waterborne disease outbreaks can occur due to various factors, such as heavy rainfall or spring runoff, improper disposal of manure, sewage, or wastewater, and issues with septic tanks, cesspools, sewers, or landfills. Problems can also arise from the failure of water treatment systems, poor sanitation in wells, and inadequate maintenance or treatment practices.1,2
Aotearoa New Zealand’s (NZ) water system is divided into two types: public and private. Public water supplies are treated and regularly tested, ensuring they meet safety standards set by the drinking water regulator, Taumata Arowai, under the Water Services Act 2021.3 There are additional protections for source waters serving more than 500 people under the National Environmental Standards for Human Drinking Water Sources (NES-DW).4 However, about 15% of New Zealanders rely on private supplies, which are unregulated and maintained by individuals or communities. These private water supplies are more common in rural areas where sources like wells or rainwater are used for drinking. Without regulatory oversight and treatment, private water supplies are vulnerable to contamination, especially by harmful microbes like Campylobacter.1
The risk of waterborne diseases is demonstrated by past outbreaks. In 2016, more than 5,000 residents in Havelock North were affected by a Campylobacter outbreak linked to contaminated community drinking water.5,6 Despite improved regulations for public water systems, there have been no additional protections for private water users, and thus rural communities continue to face higher risks of contamination.7
How Campylobacter spreads
Campylobacter is a leading cause of bacterial gastroenteritis worldwide, with different transmission routes depending on the environment. In urban areas in NZ, Campylobacter infections are mainly spread from contaminated poultry that has been eaten without sufficient cooking or has cross-contaminated other food.8,9 In rural areas, the risk is higher due to the added contribution of environmental sources such as contact with farm animals such as cattle, sheep, and poultry.10 Runoff from farms, especially during heavy rainfall or irrigation, can carry faecal matter containing Campylobacter into nearby water sources, contaminating streams, rivers, and groundwater.11–14 This contamination is particularly problematic in areas where the drinking water supply is not adequately treated, increasing the risk of infection. In areas with high concentrations of dairy cattle, the risk of contamination is particularly high, and infections tend to peak during certain periods when runoff is more likely.15
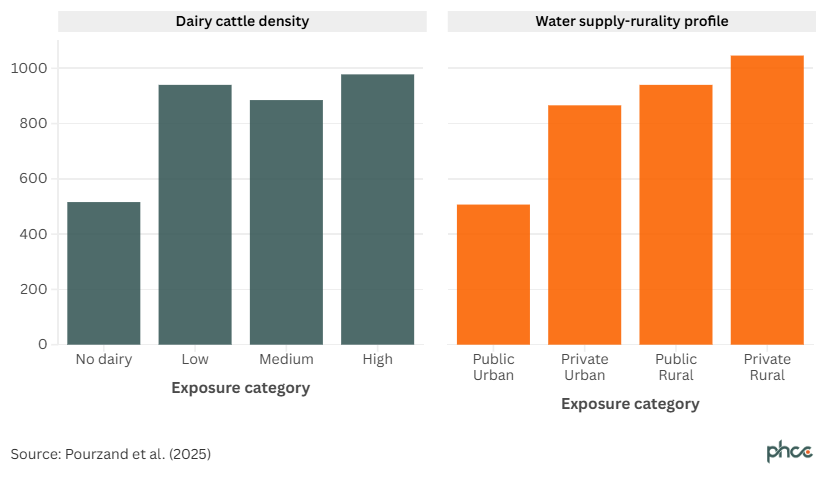
Our recent study 16 examined how the type of water supply affects Campylobacter infection rates in rural and urban areas. Campylobacteriosis notification rates are typically highest in regions with dense dairy cattle populations and rural areas relying on private water supplies. On average, areas with high dairy cattle density report an incidence of 978 cases per 100,000 people, while rural areas with private water systems see even higher rates, reaching 1,046 cases per 100,000 population (Figure 1).
Figure 1. Mean campylobacteriosis rate per 100,000 by exposure category, mean NZ rate, 2015 – 2019
Our analysis identified the following factors associated with higher infection risk:
- Having a rural private water supply: People using private water supplies in rural areas are more than twice as likely to get a Campylobacter infection compared to urban residents who have access to treated public water.
- Living in a high dairy farming area: In areas with high dairy farming, people relying on private water supplies have a 47% higher risk of contracting Campylobacter than those in areas without dairy farming.
- Being a young child: Children aged under five years living in rural areas with private water supplies are nearly four times more likely to be affected than children in urban areas with treated water.
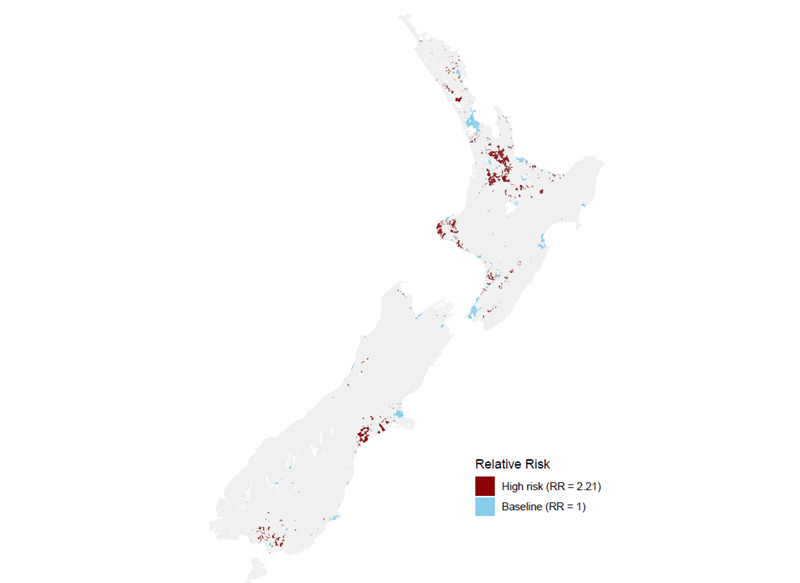
A map (Figure 2) highlighting these findings shows that regions like Waikato, Taranaki, Canterbury, and Southland—areas with both high dairy farming and private water supplies—are at greatest risk of Campylobacter. In contrast, urban areas like Auckland, Wellington, and Christchurch, where public water supplies are used and dairy farming is minimal, face much lower risk. Only a small portion of the population, about 53,546 individuals (1.13%), live in "high-risk" areas. These high-risk areas are typically rural, have high dairy cattle density, and depend on private water supplies. In contrast, most of the population (76.4%) lives in areas classified as " baseline risk". These individuals reside in urban areas with public water supplies and no dairy cattle, which are generally less vulnerable to waterborne diseases like campylobacteriosis.
Figure 2. Risk areas for campylobacteriosis
What this Briefing adds
- While contaminated chicken meat is the major source of Campylobacter infection in NZ, contaminated drinking water is also important in some settings.
- People living in regions with high dairy farming and private water sources, such as Waikato, Taranaki, Canterbury and Southland are more vulnerable to campylobacteriosis.
Implications for policy and practice
- The Ministry for the Environment, with support from Taumata Arowai and other key agencies, should expand the NES-DW to cover private water supplies to ensure that all New Zealanders, whether they rely on public or private water sources, have access to safe drinking water.
- Policymakers can use the identified high-risk areas to guide the delivery of specific interventions, such as better water quality monitoring and improved water treatment options in rural areas with high livestock densities.
- Regional councils could incorporate these findings into local environmental regulations, improving the management of water quality and reducing the risk of contamination in currently high-risk areas.
Authors details
Dr Farnaz Pourzand Department of Public Health, University of Otago, Wellington | Te Whare Wānanga o Otāgo ki Te Whanga-nui-a-Tara
Assoc Prof Tim Chambers Ngāi Tahu Research Centre, University of Canterbury | Te Whare Wānanga o Waitaha
Prof Simon Hales Department of Public Health, University of Otago, Wellington | Te Whare Wānanga o Otāgo ki Te Whanga-nui-a-Tara
Prof Michael Baker Department of Public Health, University of Otago, Wellington | Te Whare Wānanga o Otāgo ki Te Whanga-nui-a-Tara